Princeton Weekly Bulletin October 5, 1998
Why does lupus affect more women?
By Mary Caffrey|
|
|
|
|
|
The assumption that hormonal differences are behind the discrepancy in the percentage of men or women who have a specific disease has kept researchers from investigating other gender-based causes.
That thinking is the basis of a theory discussed in the August issue of Immunology Today by Jeffrey Stewart, a member of the professional technical staff in the lab of Molecular Biology Professor Martin Weigert. Stewart's work focuses on systemic lupus erythematosus, but his theory could have implications for other autoimmune diseases.
Women account for up to 90 percent of those who suffer from lupus. For years, research on the disease has focused on estrogen, with good reason: The disease primarily affects women of childbearing age, and estrogen has been shown to exacerbate the disease in lab animals. But there were problems as well. Patients with lupus had normal estrogen levels, and experiments with estrogen-laden birth control pills were inconclusive.
![]() Could
something about women, besides their hormones, be the culprit in
lupus? Stewart thinks so. His theory starts with a basic tenet of
human reproduction: the knowledge that while men have only one X
chromo-some, received from their mothers, women have two X
chromosomes, one from each parent. In each cell in a woman's body,
one of those X chromosomes is inactivated. Thus, in men each cell
has the same set of active chromosomes, while women are a
"mosaic" of two types of sets, one set being cells with functional
X chromo-somes from the mother, the others with functional X
chromosomes from the father.
Could
something about women, besides their hormones, be the culprit in
lupus? Stewart thinks so. His theory starts with a basic tenet of
human reproduction: the knowledge that while men have only one X
chromo-some, received from their mothers, women have two X
chromosomes, one from each parent. In each cell in a woman's body,
one of those X chromosomes is inactivated. Thus, in men each cell
has the same set of active chromosomes, while women are a
"mosaic" of two types of sets, one set being cells with functional
X chromo-somes from the mother, the others with functional X
chromosomes from the father.
T cell behavior
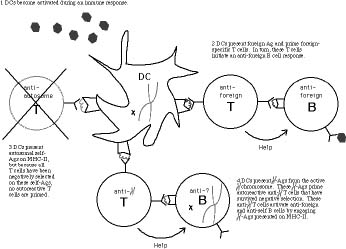
For Stewart, this mosaic could be the root of an autoimmune disease such as lupus. Human immune systems rely on the ability of T cells to recog-nize foreign cellsor "non-self" cells in the body, such as cancerous tumors, virally-infected cells or even trans-planted organs. But things can go wrong, causing a T cell to react even when there is no invader. The body has a back-up plan for this occurrence. As T cells mature in the thymus, they react with dendritic cells, which screen out the troublesome autoreactive T cells. If the autoreactive T cells fail to encounter dendritic cells, Stewart reasoned, these T cells can wreak havoc, causing the body's immune system to battle normal cells. (Thus, the term "autoimmune disease.")
Stewart's theory suggests that if a T cell is reactive only to dendritic cells with an active X chromosome from one parent, a woman who has a lop-sided number of cells with active X chromosomes from the other parent could be a candidate for an auto-immune disease. While the number of active X chromosomes from each parent in the cells of the entire female population is roughly equal, the split can vary greatly among individual women. For some, the share of active X chromosomes from just one parent can be as high as 9 in 10.
Dropped everything
In Immunology Today, Stewart pegs his theory to a statistical analysis of a combination of factors; what happens to autoreactive T cells when a woman's dendritic cell makeup includes a highly skewed share of active X chromosomes from one parent, and how likely is this scenario?
Stewart takes particular note of a series of 1997 experiments led by Tsuyoshi Sakane of St. Marianna University in Japan, which examined the link between certain T-cell clones and B cells in female lupus patients. The link between B cells and lupus had already been established. However, Sakane's research showed that it was the reaction with T cells, not neces-sarily B cells acting on their own, that caused the production of both anti-foreign antibodies (which are expected) and anti-self antibodies (which are unexpected). This peculiar auto-reactive quality of the T cells caught Stewart's attention, for he felt his theory could explain the cells' behavior.
While Stewart had been thinking about the relationship between the "mosaic" and autoimmune disease for several months, it was the publication of Sakane's results that gave him data to confirm his idea. "All the pieces fell into place," said Stewart. "X-chromo-some inactivation provided the female mosaic; the low number of dendritic cell precursors provided the skew in the dendritic cell populations; and Sakane provided the autoimmune T cells. I dropped everything and immediately began writing a manu-script."
Clincial testing at NYU
The theory has implications for diseases besides lupus. Stewart notes the high incidence of autoimmunity in those with X-chromosome aberrations, including those with Klinefelter's syndrome (males with two X chromosomes) and Turner's syndrome (females with a damaged X chromosome).
Stewart's theory is already being tested in a clinical setting. Dr. Peter Gregersen of the New York University School of Medicine, working at North Shore University Hospital on Long Island, is running tests on lupus patients that could lead to a very simple treatment for the disease.
If the critical dendritic cells are failing to recognize autoreactive T cells, Stewart said, it is possible to chemically treat a patient's dendritic cells to make the autoreactive T cells aware of the X chromosome they failed to see in development. A patient's blood would be drawn, treated and replaced, thus boosting the functioning dendritic cells that would carry out the screening duties. Stewart already has a patent pending on this procedure.
